[ad_1]
In its first week, a fertilized human egg develops into a hollow ball of 200 cells and then implants itself on the wall of the uterus. Over the next three weeks, it divides into the distinct tissues of a human body.
And those crucial few weeks remain, for the most part, a black box.
“We know the basics, but the very fine details we just don’t know,” said Jacob Hanna, a developmental biologist at the Weizmann Institute of Science in Israel.
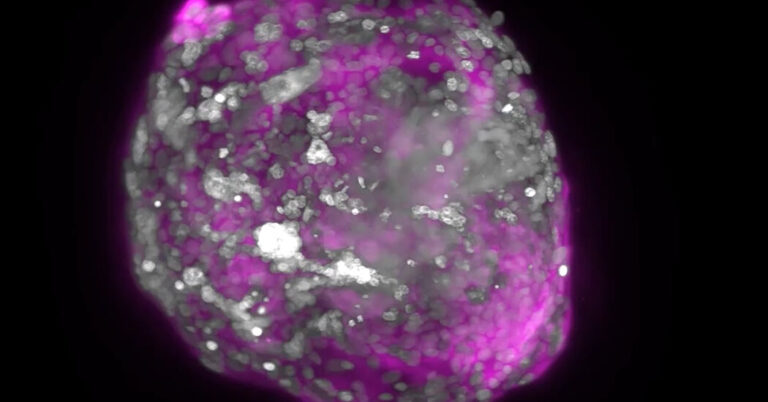
Dr. Hanna and a number of other biologists are trying to uncover those details by creating models of human embryos in the lab. They are coaxing stem cells to organize themselves into clumps that take on some of the crucial hallmarks of real embryos.
This month, Dr. Hanna’s team in Israel, as well as groups in Britain, the United States and China, all released reports on these experiments. The studies, while not yet published in scientific journals, have attracted keen interest from other scientists, who have been hoping for years that such advances could finally shed light on some of the mysteries of early human development.
Ethicists have long cautioned that the advent of embryo models would further complicate the already complicated regulation of this research. But the scientists behind the new work were quick to stress that they had not created real embryos and that their clusters of stem cells could never give rise to a human being.
“Our aims are never for the purpose of human reproduction,” said Tianqing Li, a developmental biologist at Kunming University of Science and Technology in China, who led one of the new studies.
Instead, Dr. Li and his fellow scientists hope that embryo models will lead to new treatments for infertility and even diseases such as cancer.
“We do it to save lives, not create it,” said Magdalena Zernicka-Goetz, a developmental biologist at the University of Cambridge and the California Institute of Technology, who led another effort.
For decades, the only human embryos that developmental biologists could study were specimens collected from miscarriages or abortions. As a result, scientists were left with profound questions about the start of human development. Thirty percent of pregnancies fail in the first week, and another 30 percent fail during implantation. Researchers have been at a loss to explain why a majority of embryos don’t survive.
After the development of in vitro fertilization in the 1970s, scientists began studying embryos donated from fertility clinics. Some countries banned the research, while others allowed it to proceed, typically with a 14-day limit. By then, the human embryo starts taking on some of its key features. A structure called the primitive streak, for example, organizes the head-to-foot arrangement that the body will take.
For years, the 14-day rule was a moot point because no one could keep embryos alive more than a few days after fertilization. Things became more complicated in 2016, when Dr. Zernicka-Goetz’s group and another team managed to keep embryos alive close to the 14-day mark. The embryos did not survive longer because the scientists destroyed them.
The accomplishment has led scientists to debate the possibility of allowing embryos to grow past 14 days. But even if those experiments were to become legal, they would still be hard to carry out because the supply of donated embryos is scarce.
In recent years, researchers have been looking for an easier way to study embryos: by making models of them in the lab. The scientists have taken advantage of the fact that stem cells, given the right environmental conditions, can turn into new kinds of tissues.
Adults have stem cells in only a few parts of the body. In the skin, for example, stem cells produce a range of new cells that heal wounds. In early embryos, on the other hand, all the cells have the potential to turn into a wide variety of tissues.
Last year, Dr. Zernicka-Goetz’s team and Dr. Hanna’s team used embryonic stem cells from mice to make models of embryos. Since then, they and other scientists have been trying to do the same with human embryonic stem cells.
Each team has used a different method, but they all take advantage of the same underlying biology. By the time a human embryo implants itself in the uterus, its cells have started to diverge into different types. One type of cell will go on produce the cells of the body. The other types will produce tissues that surround the embryo during development, such as the placenta. These cell types send out molecular signals to each other that are essential for their development.
The researchers coaxed stem cells to mimic some of these cell types and then mixed them together. The cells swarmed together and spontaneously organized into clusters. The cells destined to become the embryo huddled in the middle, while the other types migrated to the outside.
As the cells communicated to each other, they divided and formed new structures that resembled parts of embryos. Dr. Mo Ebrahimkhani, a developmental biologist at the University of Pittsburgh, and his colleagues observed the formation of a yolk sac in their experiment, for example. Out of the yolk sac, they even observed the development of progenitors of blood cells.
Dr. Zernicka-Goetz and her colleagues likewise watched the development of cells that resembled the precursors of eggs and sperm.
“This was absolutely thrilling,” Dr. Zernicka-Goetz said. “It’s sometimes hard to believe that these stem cells are growing into these structures.”
If scientists can create close, reliable models of embryos, they will be able to run large-scale experiments to test potential causes of pregnancy failures, such as viral infections and genetic mutations.
The models could lead to other medical advances too, noted Insoo Hyun, a member of the Harvard Medical School Center for Bioethics who was not involved in the new studies.
“Once you get the embryo models in place and you can rely on them, that can be an interesting way to screen drugs that women take when they’re pregnant,” he said. “That would be an enormous benefit.”
Dr. Hanna and Dr. Ebrahimkhani also saw a possibility of using embryo models as a new form of stem-cell treatment for diseases such as cancer.
In conventional stem-cell transplants, doctors remove blood stem cells from the bone marrow before killing cancer cells with radiation or chemotherapy. They then return the healthy cells to the body.
Unfortunately, this method does not have a high success rate. Some researchers have suggested that earlier forms of stem cells would be more likely to cure patients.
Embryo models might make it possible for doctors to turn back time. Researchers would take skin cells from a patient and douse them with chemicals to put them into a stem-cell-like state. With other chemical baths, those stem cells could then be turned into an embryo model, which could in turn develop into the early blood cells the patient needs after a transplant.
Alysson Muotri, a developmental biologist at the University of California San Diego who was not involved in the new studies, cautioned that the new studies demonstrated only a preliminary step. For one thing, while the techniques sometimes resulted in embryolike clusters, they often failed.
“The work is in very early stages, and the current methods are far from reliable,” Dr. Muotri said.
[ad_2]