A Random Influx of DNA from a Virus Helped Vertebrates Become So Stunningly Successful
Insertion of genetic material from a virus into the genome of a vertebrate ancestor enabled the lightning-quick electrical impulses that give animals with backbones their smarts
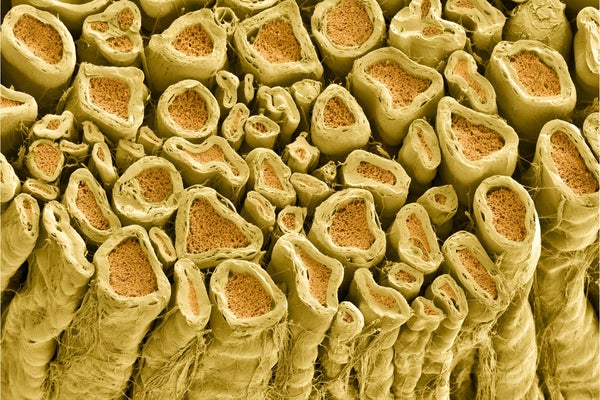
White myelin sheaths around rat nerve fibers.
NIH/Image Point FR/BSIP SA/Alamy Stock Photo
Charles Darwin proposed that evolution is driven by gradual variations in organisms that have a survival advantage in a changing environment. But University of Maryland evolutionary biologist Karen Carleton says that scientists have long grappled with the quandary that “evolution can happen abruptly, as described by Steven Jay Gould in [the theory of] punctuated equilibrium.” The question has always been: How does this happen?
A case in point is the sudden appearance of myelin, the multilayered sheath on nerve fibers that transformed the way neural impulses are conducted and turbocharged the transmission speed of these impulses. Myelin appears suddenly in vertebrates, animals with backbones that arose 500 million years ago. Not a trace of it is found in the ancestral line that preceded the arrival of vertebrates. A new study in the journal Cell provides an answer to this long-standing puzzle: the genetic instructions to make myelin were slipped into our vertebrate ancestor’s DNA by infection with a virus.
Myelin is arguably the most significant advance in nervous systems that ever occurred in the animal kingdom. The great boost in speed of information transmission over long distances in the body is largely responsible for the dramatic leap in cognitive ability in vertebrates, not to mention speed of movement and agility in dogs, dolphins and people, for example, when compared with invertebrates such as slugs, worms and starfish. Lacking myelin, neurons in invertebrates are clustered into groups (ganglia) situated near the body structures they control or that provide sensory input. There are ganglia next to every swimming leg in a shrimp’s tail, for example, but in vertebrates, neurons are massed together into one enormous central assembly, the brain. The concentration of billions of neurons into a brain enabled cognitive capabilities well beyond those of invertebrates.
On supporting science journalism
If you’re enjoying this article, consider supporting our award-winning journalism by subscribing. By purchasing a subscription you are helping to ensure the future of impactful stories about the discoveries and ideas shaping our world today.
Curiously, myelin is wrapped around nerve fibers by entirely different cells in the body (Schwann cells) than in the brain (oligodendrocytes). If myelin evolved independently in the peripheral and central nervous system, transmission delays in either part of the system would undermine the advantage, like a slow Internet connection hobbling a high-speed computer. But myelin appears fully formed simultaneously in the brain and body with the evolution of vertebrates. (One exception is the lamprey eel, the most primitive living vertebrate, which has no myelin.) All of this raises the question: Where did myelin come from?
“Myelin evolution is an important mystery and is totally understudied,” says myelin researcher Robert Gould at the Marine Biological Laboratory’s Whitman Research Center, who was not involved in the new research. The study reports the involvement of what is called a retrovirus in precipitating the appearance of myelination. Gould says that involvement of retroviral RNA in myelination is a surprise—one that should have important implications for myelin-related diseases, such as multiple sclerosis.
The central dogma in molecular biology holds that information flows from DNA in the cell nucleus and is carried outside the nucleus to the cytoplasm, the cell’s liquid interior, by another molecule, messenger RNA. The messenger RNA transports a copy of the genetic code for a specific protein to deliver to specific structures in the cytoplasm that synthesize that protein. Viruses cannot make proteins on their own. Instead they hijack the molecular machinery of the cells they infect to make all the viral proteins and enzymes needed to generate new viruses.
Retroviruses such as the HIV virus carry out this genetic hacking by reversing the sequence of the gene readout. They inject their RNA into the cells they infect, which serves as the code to make viral proteins. That RNA is converted into DNA and, like malicious code in a software program, gets inserted into the cell’s genome. When the cell reads out that rogue DNA code into RNA, it unwittingly makes the foreign viral proteins.
Dreadful infections and cancers are caused by viruses corrupting our genome, but sometimes a viral infection has unexpected benefits, and mutant fragments of viral genes get permanently fixed into the DNA of organisms and can be passed on for generations. These snippets of foreign DNA typically no longer make virus proteins. Still, they have a powerful influence on what genes are read out to make proteins by binding to DNA regions next to genes and to proteins in the nucleus that control whether or not a gene is expressed. Astonishingly, 40 percent of the DNA in mammals consists of remnants of these retroviral infections.
Myelin biologist Robin Franklin of the Wellcome Genome Campus in England and colleagues report in the new study that they have identified a retroviral element in all vertebrates except lampreys. The researchers have given this insertion into the genome of the common ancestor of vertebrates millions of years ago the name RetroMyelin. They have shown that it stimulates the synthesis of proteins that are essential to making myelin in both the central and peripheral nervous system.
When they blocked RetroMyelin in mouse cells in culture—and in zebrafish larva and tadpoles—myelin largely failed to form. Further experiments revealed how blocking RetroMyelin stymied myelin production. A key protein in myelin called MBP is essential to form the myelin sheath. The formation of myelin takes place as a long, tentaclelike extension from a cell called an oligodendrocyte envelops the nerve fiber.
To carry out this process, MBP on the inner surface of the oligodendrocyte’s cell membrane pairs up with the same type of molecule on the inner membrane of the oligodendrocyte’s elongated tentacle that wraps around a fiber. Binding to each other, MBP zips both interior surfaces of the membranes together like a folded piece of tape sticking to itself, and this squeezes out all the cytoplasm to form a compact sheath with high electrical resistance. The researchers showed that RetroMyelin latches onto a protein called SOX10, a transcription factor that activates the reading of DNA for the MBP gene. RetroMyelin stimulates SOX10, and in response the cells begin generating large amounts of MBP to make myelin.
“This is a very interesting study, which identifies an important factor in myelination,” says Klaus-Armin Nave, a myelin researcher at the Max Planck Institute for Multidisciplinary Sciences in Göttingen, Germany, who was not involved in the research. “But viewed rigorously, the conclusion that this retroviral infection was the switch that turned on myelination in vertebrates is based on correlation.” Myelination is a very complex process, he says, which must require many different proteins and mechanisms of gene regulation. The mystery of myelin may require further sleuthing: there is, for instance, little evidence of MBP in the ancestors of vertebrates. “Where did MBP come from if there is no DNA sequence for it in prevertebrates?” Robert Gould ponders. “Final proof,” Nave says, “would be to introduce the retroviral gene into lampreys and see if they form myelin.”
Retroviruses can be a powerful engine of evolution, and myelin appears to be one of the most remarkable examples. “It does make sense that a retrovirus might be involved,” Carleton says. One hundred years after the discovery of retroviruses and two centuries after a 22-year-old naturalist embarked on his five-year sailing journey around the world, molecular biology is now tackling the puzzle that Darwin grappled with in his momentous theory of evolution by illuminating how some traits seemingly appear out of nowhere.