
[ad_1]
On a recent visit to the Impressionist gallery at the Art Institute of Chicago, I found myself drawn to an exceptionally vibrant Renoir painting called Young Woman Sewing.
The helpful museum placard informed me that “cobalt blue dominates the image,” and I chuckled, not because I am an artist but because I am an astrophysicist; I study how the elements we find on Earth come from stars, supernovae and other astrophysical phenomena. While the elements and their cosmic origins are something I think about every day, for the first time I found myself thinking about how some elements made their way from the palette of the universe to the palette of an artist. So an ordinary statement in the context of art ended up serving as an extraordinary reminder to me that human beings, our various pursuits and the natural world are all fundamentally interconnected.
My curiosity triggered, I dug into the chemical compositions of other pigments and materials used by artists, and realized that just four of them—charcoal, cobalt blue, cadmium yellow and helium—can provide an expansive tour of the different ways in which our universe creates new elements.
But the origins of the elements are only the beginning of their story. The exploitation of humans and of our planet’s limited resources is the untold, sadder part. Charcoal production contributes to forest degradation as well as climate change through the production of greenhouse gases. Cobalt mining is being carried out under unconscionably hazardous conditions in the Democratic Republic of the Congo. Cadmium is a rare element that the European Union considered banning in pigments because it is toxic to both humans and animals, and mining and smelting have dispersed it into the environment and the food chain. We are facing a global shortage of the liquid helium that keeps the magnets in MRI machines running. Our need for these elements and what they make besides art is important to keep in mind, even as their use gives us so much beauty.
Carbon
Charcoal has been used in art since ancient times. It is largely pure carbon and readily produced from burnt wood. In a poetic parallel to charcoal’s birth in fire, most of the carbon in the universe is synthesized in the cosmic furnace of stars through the fusion of helium nuclei.
The Grotte Chauvet–Pont d’Arc in southern France contains some of the oldest cave paintings in the world, created by our prehistoric ancestors roughly 30,000 years ago. Researchers believe these early artists selected charcoal because it was the perfect medium, ideal for the smudging and blending techniques used in cave paintings.
Cobalt
As chemistry has developed, so has our discovery of new elements, and this has shaped art as we know it. Among the elements identified from already existing minerals and ores was the silvery metal cobalt, discovered by chemist Georg Brandt in 1739.
This element takes its name from the German word kobelt, signifying kobolds—gnomes and goblins thought to haunt mines. It owes its ominous name to the corrosiveness of the minerals (often containing arsenic) it is associated with, which was so hazardous to miners that they thought it must have been placed in the mines by malicious subterranean beings.
We now know that cobalt comes not from deadly kobolds but from stellar death—specifically thermonuclear supernovae and core-collapse supernovae. Thermonuclear supernovae are explosive deaths of white dwarfs caused by runaway nuclear reactions. Core-collapse supernovae, on the other hand, arise from massive stars. Here, the collapse of the iron core generates a shock wave that blows up the entire star, forging elements like cobalt in the process through the explosive burning of silicon.
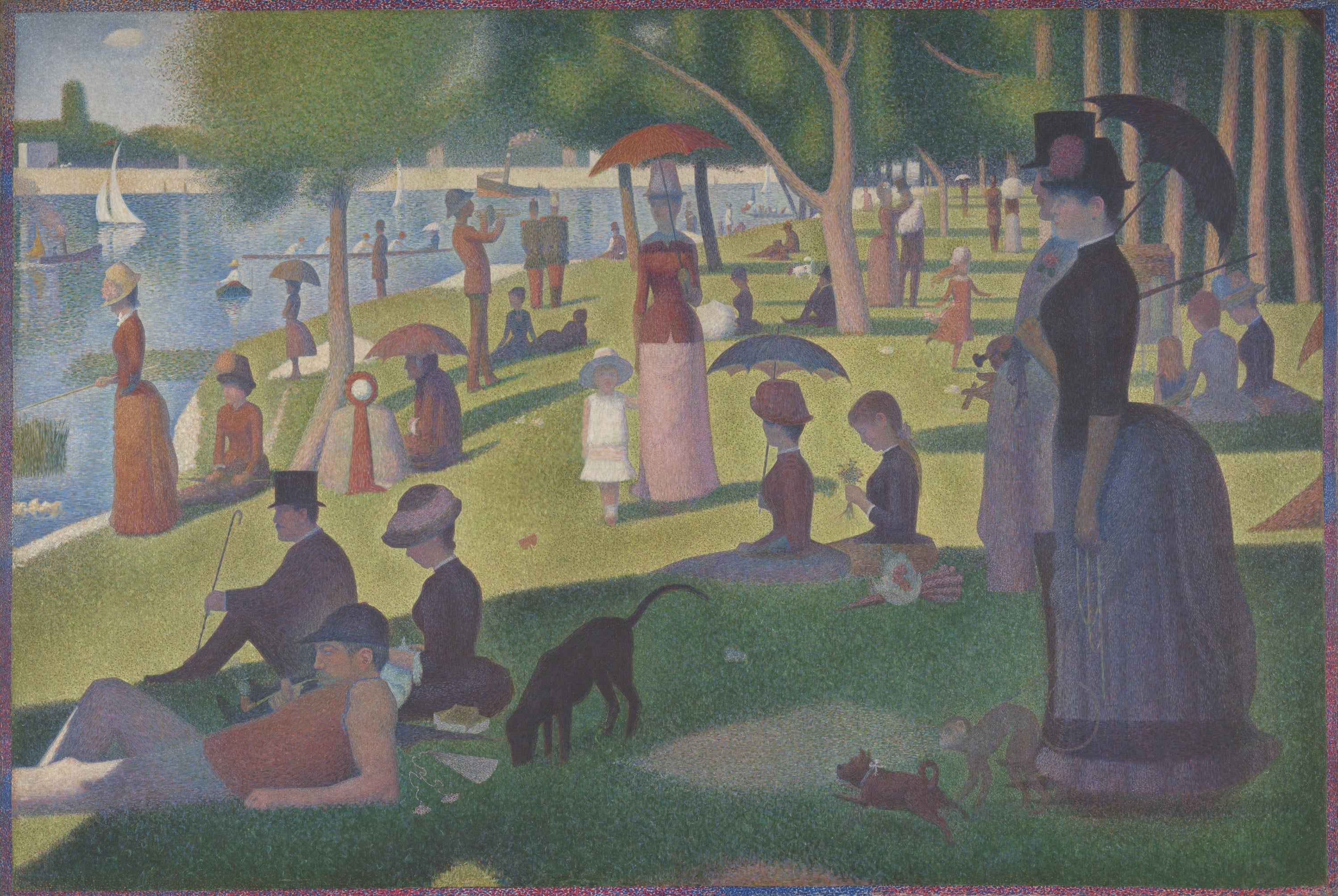
Eons later, in 1739, Louis-Jacques Thénard discovered how to make cobalt aluminate, better known as cobalt blue. Pierre-Auguste Renoir and Édouard Manet are among the many Impressionists who have used this color in their work, as did Georges Seurat, when he branched out from Impressionism to create Pointillism and painted A Sunday on La Grande Jatte. It hangs in the Art Institute of Chicago just a few steps away from the Renoir painting that first caught my eye.
Cadmium
Producing elements heavier than iron, like cadmium, requires environments rich in neutrons that are captured by lighter “seed” nuclei. This can happen slowly over a long period of time in a low-mass star, or within seconds during the highly violent merger of two neutron stars.
By the time cadmium was discovered, chemists seemed to already realize its art potential. In 1817 Friedrich Stromeyer discovered cadmium by reducing a yellow-colored substance deposited in the chimneys of a zinc smelting factory. While studying the new element, he ended up creating the bright yellow solid cadmium sulfide, remarking that it “promises to be useful in painting.”
Cadmium pigments were expensive. Among the artists who could afford to use them was Claude Monet. He used cadmium yellow in several Impressionist works like The Artist’s House at Argenteuil, Bordighera and Stacks of Wheat (Sunset, Snow Effect) from his 1890–91 Grainstacks series. All of these also hang in the Art Institute of Chicago.
Helium
Helium’s origins go back to just a few minutes after the big bang, when hydrogen, helium and a bit of lithium were produced during what is called big bang nucleosynthesis. As the universe expanded and cooled, protons and neutrons were able to assemble into nuclei, producing the lightest elements of the periodic table, and setting the stage for the birth of stars and the synthesis of new elements.
But it may be a surprise that the second element of the periodic table was discovered after elements like carbon, cobalt and cadmium. In fact, the element was discovered in the sun before it was found on Earth, as it makes up only 0.0005 percent of Earth’s atmosphere.
By the 1800s, scientists had realized that by splitting sunlight into its spectrum they could identify absorption and emission lines corresponding to the elements present in the solar atmosphere. Astronomer Pierre Jules Janssen traveled to India in 1868 to observe the light from the solar corona during a total solar eclipse and found a previously unseen and very faint emission line in the yellow region. Scientists later concluded that the line corresponded to an unknown element present in the sun. That is why helium gets its name from Helios, the Greek sun god.
Today helium finds use in contemporary art. Karina Smigla-Bobinski’s ADA is an excellent example—a kinetic sculpture consisting of a helium-filled clear balloon studded with charcoal sticks. The balloon bounces around a room with white walls, guided by the visitors to the installation, and covers the walls with interesting patterns.
By telling tales about stardust, I hope we can remind ourselves that we live in an interconnected and beautiful world, full of rare and precious elements. It is our duty to treat it, and each other, with care and respect. Not only are we stardust, but we reshape stardust every time we create something new, so let’s create wonders.
This is an opinion and analysis article, and the views expressed by the author or authors are not necessarily those of Scientific American.
[ad_2]