[ad_1]
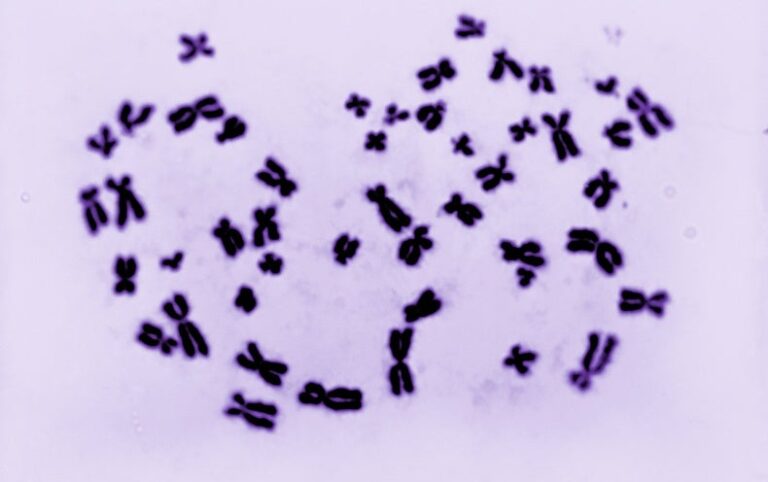
Most people have two sex chromosomes, either two X’s or an X and a Y, which give rise to female or male biological attributes on a spectrum. Studies suggest these chromosomes also have much broader effects, contributing to processes that include immune system function, neuronal development, disease susceptibility and reactions to drugs. But scrutinizing the specific role of X and Y chromosomes is challenging. With current tools, it is difficult to disentangle the effects of genes versus hormones, for example.
Now scientists have devised a tool that could overcome this obstacle—by generating XX and XY cells from a single person for the first time. This unique set of cells could help researchers tackle long-standing questions about how sex chromosomes affect disease and the role they play in early development.
“This is a really cool set of cell lines,” says Barbara Stranger, a professor of pharmacology at Northwestern University, who was not involved in this study. “We’ve had cell lines from males and females before, but the fact that they’re coming from same person with just the same sex chromosome difference—it’s a big step.”
Benjamin Reubinoff, a professor of obstetrics and gynecology at the Hadassah Medical Center in Israel, and his team began the project to overcome barriers facing investigations of sex differences in humans. Currently there are two major ones, according to Reubinoff: the difficulty of separating chromosomal and hormonal effects and the inability to pinpoint the effects of X and Y chromosomes while ruling out contributions from the rest of a person’s genetic makeup. “The main reason for doing this study was the lack of a good model to study differences between males and females in humans,” Reubinoff says. “There have been animal models, but a model in humans was not available.”
To create such a model, Reubinoff, his former M.D. and Ph.D. student Ithai Waldhorn and their colleagues first obtained white blood cells previously collected from a person with Klinefelter syndrome, a condition in which male individuals are born with an extra X chromosome. The cells came from the repositories of the Coriell Institute for Medical Research, where people donate samples for use in a wide range of biomedical research projects. The donor had a rare “mosaic” form of the condition, in which some of their cells had three sex chromosomes (XXY), some had two X chromosomes, and some had one X and one Y. The researchers reprogrammed all three cell types into induced pluripotent stem cells, which have the capacity to self-renew and to develop into neurons, muscle cells or other cell types.
Ultimately the team generated XX and XY cells that—apart from their sex chromosomes—were genetically identical. The researchers then conducted a series of experiments replicating findings from prior studies with other models. For example, they confirmed previously reported differences in genes that were turned on in XX or XY cells. They also coaxed their stem cells to develop into immature versions of neurons and found evidence of previously reported sex differences in early neural development. “It was reassuring to see that the model really shows differences between the sexes that were reported from other systems,” Reubinoff says. The findings were published last month in Stem Cell Reports.
“This is a very well-designed study that validates the notion that sex differences start early in development—and that they depend on the sex chromosomes because that’s the only thing that can account for those differences,” says Nora Engel, a professor of cancer and cell biology at Temple University, who was not involved in this work.
In the past, researchers have probed the effects of sex chromosomes in animals using the “four core genotypes” mouse model, which includes modifying a gene called Y(Sry). This region of the Y chromosome contains instructions for developing testes, and the changes create animals with XX chromosomes and testes or XY chromosomes and ovaries. Animals with the same sex organs but different chromosomes enable scientists to isolate the effects of sex chromosomes from the effects of sex hormones, which are secreted by the reproductive organs. The mouse model was transformative for the field of sex difference research, Stranger says. Being able to move this research into humans “is really neat,” she adds.
“I think this is going to open up avenues for new research,” says Jessica Abbott, a senior lecturer in eukaryote evolutionary genetics at Lund University in Sweden, who was not involved in this research. Abbott notes that it will be important to derive XX and XY stem cells from another person to see how much variation there is between people—which will help determine how generalizable findings from these cells are to the broader population.
Cells, of course, cannot model the entire human body or interactions between organs—at least not yet. But Reubinoff notes that with the development of new techniques such as microfluidic “body-on-a-chip” systems that model the connections between cells from different organs, scientists may be able to address a broader array of research questions in the future. For now, Reubinoff is excited about the experiments that will be possible with the stem cells alone. “You have a tool that you can, at least theoretically, use [indefinitely to] generate any cell type and develop models for various types of diseases,” he says. “The model we developed opens wide horizons.
[ad_2]